EU TPD Compliance for Nicotine Pouches: The COO’s Blueprint for Market Entry
This document is the exact 12-step Standard Operating Procedure you need to master EU TPD compliance for nicotine pouches and turn a complex regulatory hurdle into a straightforward, profitable launch plan. I’ve personally used this blueprint to guide dozens of partners, from regional distributors to national retail chains, through this process. Follow these steps, and you will eliminate ambiguity and get your brand onto shelves faster.
The goal here isn’t just to get a certificate. It’s to achieve swift, profitable market access in your target EU countries. You will treat the Tobacco Products Directive (TPD) as a manageable process, not an insurmountable barrier.
Objective & Prerequisites: Laying the Foundation for Compliant Market Entry
Before we begin, you must have your foundation in order. This is non-negotiable.
- Secure your legal entity. You need a registered business with a valid VAT number within the European Union.
- Finalize your target markets. Decide on the initial list of EU member states you will launch in.
- Allocate your budget. You must account for notification fees, laboratory testing, and professional translation services.
- Designate a “Responsible Person.” Assign a single point of contact inside your company. This person will be the dedicated liaison with us at EastSnus and, if required, the national competent authorities.
Phase 1: Compliance-by-Design (Product & Strategy)
This is where you win or lose the game. Integrating compliance directly into your product design phase prevents the most common, costly, and time-consuming mistakes. Get this right, and everything else flows smoothly.
Step 1.1: Conduct Member-State Specificity Analysis
You must understand that the TPD sets a baseline, but individual EU member states add their own rules on top. Your first operational task is to map these variations for each target country.
Focus your analysis on specific national regulations covering flavors, packaging dimensions, warning label languages, and, critically, excise taxes or any specific national levies.
Pro Tip: Design your product and packaging to meet the requirements of your strictest target market first. This creates a “master” compliant product. This master SKU can then be deployed much faster across other, less-restrictive EU states with minimal changes, saving you time and money.
Step 1.2: Define a TPD-Compliant Product Formulation
Your product formula must be compliant from day one. There is no room for error here.
- Lock in nicotine content. The maximum legal limit is 20 mg of nicotine per individual pouch. This is a hard limit.
- Validate all ingredients. We provide you with a pre-vetted library of compliant, high-quality ingredients. Every single one must be validated against the TPD Article 7 list of prohibited additives, which includes vitamins, caffeine, and other stimulants.
- Finalize specifications. Lock down the exact pouch weight, the product’s pH level, and the nicotine release profile. These are all data points required for your submission.
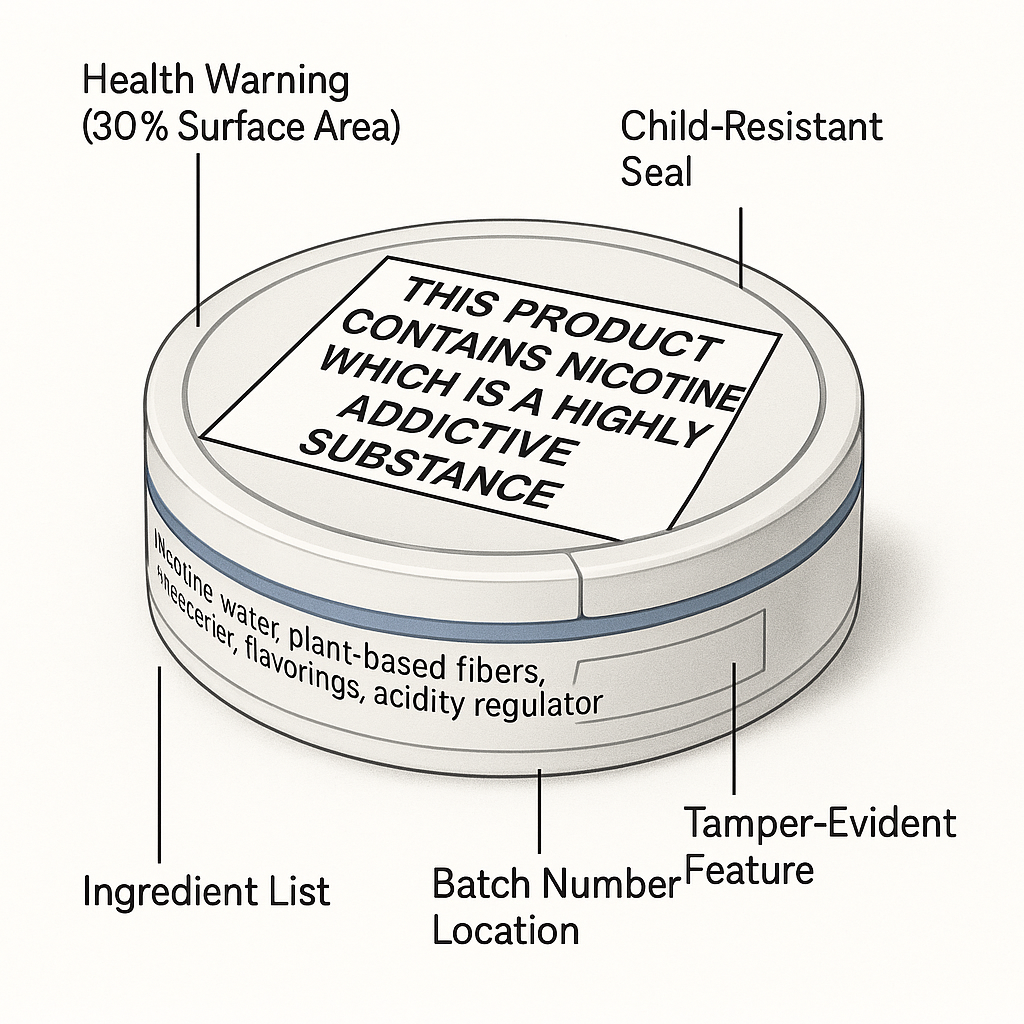
Step 1.3: Engineer Compliant Packaging & Labeling
Your packaging is a critical compliance tool. It must be engineered, not just designed.
- Incorporate health warnings correctly. This includes the exact text, size (covering 30% of the two largest surfaces), and placement as required by each target country.
- Ensure child-resistant and tamper-evident features. This is a mandatory safety requirement for consumer products. We build this into our standard can designs.
- Integrate traceability data. You must have dedicated space on your artwork for batch numbers and “best before” dates. This is essential for quality control and any potential recall.
Checklist for Phase 1 Completion:
- [ ] Target EU Countries Finalized & Confirmed
- [ ] Member-State Specificity Report Complete
- [ ] Product Formulation Confirmed (Nicotine <= 20mg/pouch)
- [ ] All Ingredients Vetted Against Prohibited Lists
- [ ] Compliant Packaging Artwork Approved by All Stakeholders
Phase 2: Executing EU TPD Compliance for Nicotine Pouches: The Core Notification Process
With a compliant product designed, we move to the official submission phase. Precision, accuracy, and meticulous documentation are your keys to success here. This is a purely process-driven stage.
Step 2.1: Compile the Technical Dossier
The Technical Dossier is the master file that proves your product’s compliance. We manage the heavy lifting here, but you need to understand what goes into it.
This dossier includes the detailed product recipe, full ingredient specifications with supplier data, and comprehensive toxicological data for all ingredients and emissions from the final product. It also contains a full description of Our Compliant Production Process and our quality control mechanisms. A complete dossier is the foundation for a smooth process in achieving EU TPD compliance for nicotine pouches.
How-To Demo: We recently guided a client targeting both Germany and Sweden. Instead of treating them as separate projects, we ran them in parallel. Our team prepared two country-specific notification packages simultaneously from a single master dossier, cutting their total administrative and preparation time by 50% and enabling a perfectly synchronized launch in both key markets.
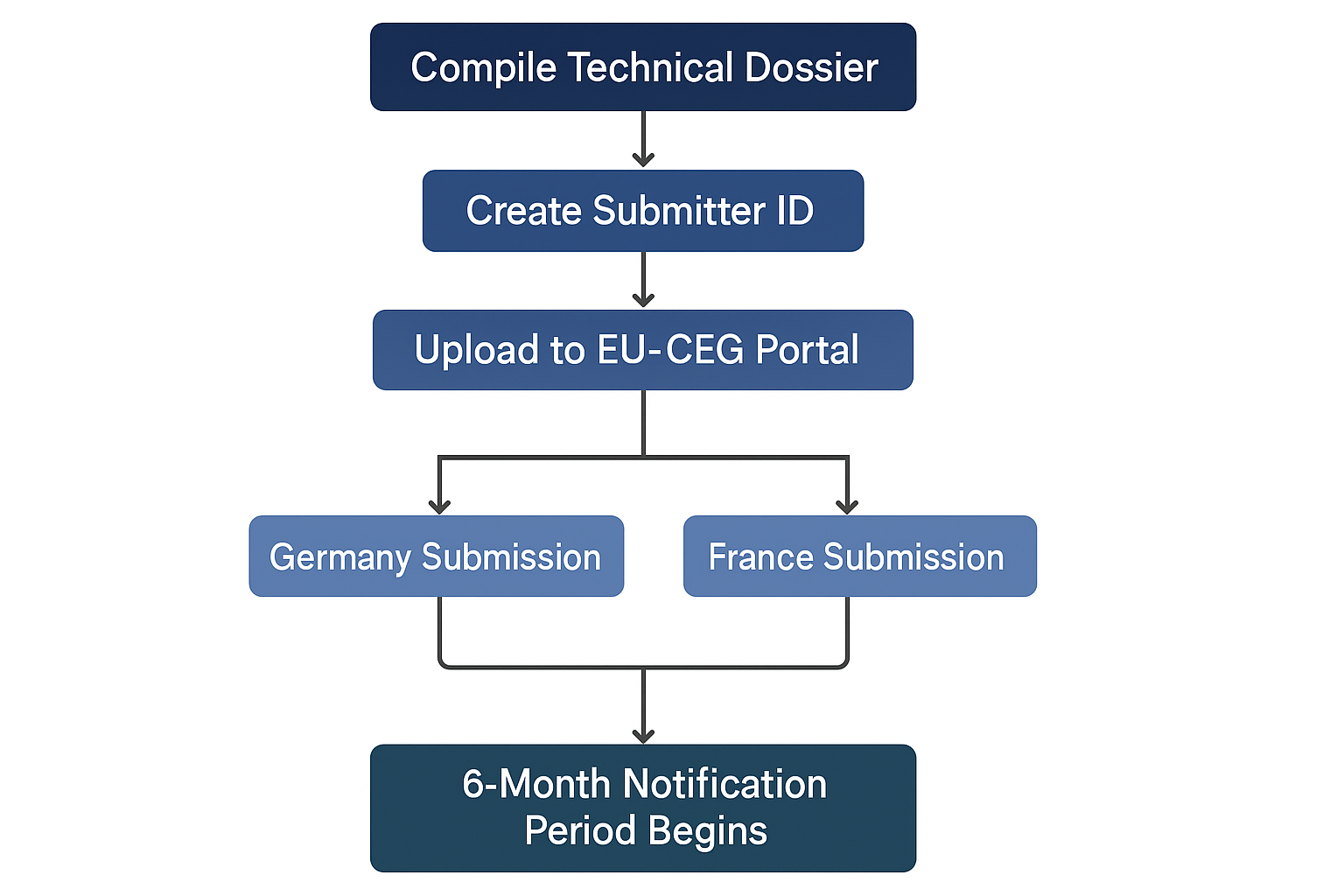
Step 2.2: Master the Submission Process via EU-CEG
All notifications are submitted through a single European portal.
- Create a submitter ID. The first step is to register your company on the EU Common Entry Gate (EU-CEG) portal. We can guide you through this process. You can find more information directly from the European Commission to the official EU-CEG portal information page.
- Upload the dossier. The complete Technical Dossier is uploaded for each individual SKU and for each target member state. A single product sold in three countries requires three separate submissions.
- Pay the notification fees. You must pay the national fees for each submission. This payment officially triggers the 6-month pre-market notification period. Your product cannot be placed on the market until this period is over.
Checklist for Phase 2 Completion:
- [ ] Technical Dossier Compiled & Verified by EastSnus
- [ ] EU-CEG Submitter ID Created and Active
- [ ] Dossier Submitted for All Target SKUs/Countries
- [ ] All National Notification Fees Paid & Confirmed
- [ ] 6-Month Notification Period Start Date Logged
Phase 3: Go-to-Market & Ongoing Surveillance
Securing your notification is a major milestone, but compliance is an ongoing operational responsibility. A professional operation prepares for market entry and long-term success.
Step 3.1: Align Production & Supply Chain with Notification Timelines
This is a simple but critical logistical step that many new brand owners get wrong.
- Schedule your first production run. This run must be scheduled to complete after the 6-month notification period officially ends.
- Do not ship prematurely. Product cannot be shipped to your EU distribution center or retailers before the legal market entry date.
Pro Tip: A premature shipment is considered an illegal product. It can be seized by customs, leading to a total loss of the inventory and the risk of significant fines and penalties. We mitigate this by aligning our production schedules directly with your EU-CEG notification status, ensuring your product is ready the moment it becomes legal to sell.
Step 3.2: Implement Post-Market Surveillance Systems to Maintain EU TPD Compliance for Nicotine Pouches
Once your product is on the market, you are responsible for monitoring it. Maintaining your EU TPD compliance for nicotine pouches status requires active vigilance.
- Collect market feedback. Establish a formal system for collecting, reviewing, and acting upon feedback regarding product quality and safety.
- Maintain batch traceability. You must be able to trace every single can from raw materials through to the point of sale. Our production system embeds this from the start.
- Prepare a Product Recall Plan. Having a formal, documented recall plan is the mark of a professional operation. This is often requested by authorities during routine regulatory audits and is required under general product safety regulations to EU General Product Safety Regulation information.
Checklist for Phase 3 Completion:
- [ ] First Production Run Scheduled for Post-Notification Period
- [ ] Shipping & Logistics Aligned with Legal Market Entry Date
- [ ] Batch Traceability System Active and Tested
- [ ] Market Surveillance & Feedback Protocol in Place
- [ ] Formal Product Recall Plan Documented
The EastSnus TPD Compliance Checklist: Your Action Plan
Use this master checklist to track your progress from start to finish. This is your operational blueprint. Print it out and put it on your wall.
- Phase 1: Design
- [ ] Target EU Countries Finalized
- [ ] Member-State Specificity Report Complete
- [ ] Product Formulation Confirmed (<= 20mg Nicotine)
- [ ] Compliant Packaging Artwork Approved
- Phase 2: Execution
- [ ] Technical Dossier Compiled & Verified by EastSnus
- [ ] EU-CEG Submitter ID Created
- [ ] Dossier Submitted for All SKUs/Countries
- [ ] Notification Fees Paid & Confirmed
- Phase 3: Go-to-Market
- [ ] Production Scheduled Post-Notification Period
- [ ] Batch Traceability System Active
- [ ] Market Surveillance Protocol in Place
This is the exact process we execute for our partners. It’s a proven system for achieving EU TPD compliance for nicotine pouches efficiently and correctly.
If you are ready to stop working for other brands and start building your own, this is your path. When you are ready to review your plan, schedule a direct consultation with my team. We will help you turn this blueprint into a market reality.
Book your TPD strategy session here: https://eastsnus.com/contact/
